The natural water oxidation catalyst found [32] in green plants is based on a tetrameric manganese cubane structure that shuttles between the +2 and +3 oxidation states. Other waste substrates, such as aqueous ethanol or biomass, can be considered along these lines and used as substrates for H2 formation. We are at the beginning of this adventure and it is unclear whether sufficient funding will be made available to meet the scientific demands. 2 By contrast, the photochemical dehydrogenation of waste materials is both viable and economical, provided adequate stocks of the waste compound are available and that the entire process is carried out locally. : Path to a Renewable Future, Water Oxidation at Electrodes Modified with Earth-Abundant Transition-Metal Catalysts, Artificial light-harvesting arrays for solar energy conversion, Enhancement of hydrogen production using photoactive nanoparticles on a photochemically inert photonic macroporous support, Synthesis, Characterization, and Reactivity of Functionalized Trinuclear Iron-Sulfur Clusters - A New Class of Bioinspired Hydrogenase Models, Reactivity of a Series of Isostructural Cobalt Pincer Complexes with CO
However, the efficiency of this method, which was developed as part of LightChEC, is not yet able to match the efficiency of PEC cells. Of the many thousands of examples where sacrificial redox reagents have been used to isolate the required catalytic step, only a small handful have been successfully carried through to the next stage. Most of the hydrogen atoms can be extracted from glycerol in this manner and converted into collectable H2. Unfortunately, most of the effective materials are based on very expensive and rare elements, although Co3O4 stands out as being a useful alternative. Furthermore, the system uses only 1per cent by volume ethanol in water and, as such, could be applied to extract the residual ethanol from biofuel production after the distillation step. Reduction to Formate, Inhibiting the Hydrogen Evolution Reaction (HER) with Proximal Cations: A Strategy for Promoting Selective Electrocatalytic Reduction, Electrochemical catalysts to meet the challenge for sustainable fuel production from renewable energy, Addressing the Reproducibility of Photocatalytic Carbon Dioxide Reduction, Atomically Dispersed Mo Supported on Metallic Co Artificial photosynthesis aims to mimic its natural role model. (Online version in colour.). We are familiar with fossil fuels, primarily coal and petroleum (both liquid and gas), formed from the fossilized remains of ancient plants and animals over some hundreds of millions of years.
This reaction does not compete effectively with back electron transfer from TiO2 to the dye. O) The exothermic conversion of quadricyclane to norbornadiene occurs only in the presence of a catalyst.Download figureOpen in new tabDownload PowerPoint. If not, is it possible to harvest solar energy from the desert and transport the generated electricity several thousands of miles to the UK? the molecular reaction mechanism, thereby helping to make the production of energy via artificial photosynthesis commercially viable at some point in the future. 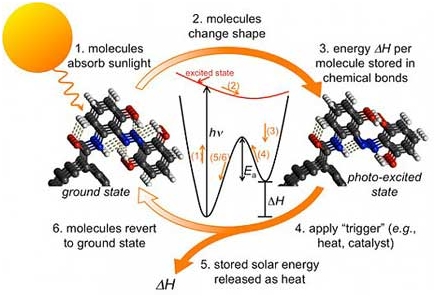 Oxygen evolution occurs at a selective catalyst prepared from cobalt phosphate. To overcome the technical problems associated with the development of a national solar fuels industry demands the identification of new protocols for the photochemical production of carbon-based fuels and for the recovery of CO2 from the atmosphere. Nonetheless, these cycles are important as a means by which to overcome the problems associated with collection and storage of the fuel. There are, indeed, few cases of O2 evolution from water where a reversible redox shuttle is involved. +, Molecular Systems for Solar H It is considered that in excess of almost one billion tonnes of ammonia are formed each year via reduction of atmospheric nitrogen by nitrogenase enzymes. Do we have sufficient sunlight in the UK to drive such technology? Table 2.Comparison of the observed rates and quantum efficiencies of O2 evolution observed for various metal oxide catalysts under photochemical conditions. There can be little doubt that these problems will grow in importance over the coming years, not least because of population growth and an escalation in our energy usage; the latter is expected to double before 2050 (as discussed by Lewis& Nocera [1]). Figure 3. The harvesting of sunlight using solar cells and the electrolysis of water are processes that (can) in principle be carried out separately. Therefore, the process does not involve solar cells. Although numerous photosystems have been set up that evolve H2 or O2 under illumination, there seems little likelihood that such approaches can be made practical. Cycle proposed for the photochemical dehydrogenation of glycerol as produced as a side-product during biodiesel manufacture.
Oxygen evolution occurs at a selective catalyst prepared from cobalt phosphate. To overcome the technical problems associated with the development of a national solar fuels industry demands the identification of new protocols for the photochemical production of carbon-based fuels and for the recovery of CO2 from the atmosphere. Nonetheless, these cycles are important as a means by which to overcome the problems associated with collection and storage of the fuel. There are, indeed, few cases of O2 evolution from water where a reversible redox shuttle is involved. +, Molecular Systems for Solar H It is considered that in excess of almost one billion tonnes of ammonia are formed each year via reduction of atmospheric nitrogen by nitrogenase enzymes. Do we have sufficient sunlight in the UK to drive such technology? Table 2.Comparison of the observed rates and quantum efficiencies of O2 evolution observed for various metal oxide catalysts under photochemical conditions. There can be little doubt that these problems will grow in importance over the coming years, not least because of population growth and an escalation in our energy usage; the latter is expected to double before 2050 (as discussed by Lewis& Nocera [1]). Figure 3. The harvesting of sunlight using solar cells and the electrolysis of water are processes that (can) in principle be carried out separately. Therefore, the process does not involve solar cells. Although numerous photosystems have been set up that evolve H2 or O2 under illumination, there seems little likelihood that such approaches can be made practical. Cycle proposed for the photochemical dehydrogenation of glycerol as produced as a side-product during biodiesel manufacture.
Cost-effectiveness and conversion efficiencies are of major importance but so too is storage.
The product, to be classified as a fuel, must be storable. It is clear that solar energy conversion could be invaluable in this area. A major advantage for biofuel relates to the fact that it can be produced from any carbon source that itself can be replenished rapidly. Illustration of the system developed by Nocera et al. A significant fraction of our total electricity production is used for industrial and domestic heating and/or cooling. The supply of secure, clean, sustainable energy is a major scientific and technological challenge that must be solved within the next few decades in order to avoid catastrophic changes in society. PEC cells currently have an efficiency of around five percent. Rh2O3 and MnO2 as being suitable materials for the anode.
S Again, there are problems to identify selective catalysts, although Au nanoparticles look promising in this field.
3 Nonetheless, this is a valuable practical application of solar energy conversion. One substrate that springs to mind is glycerol, a by-product of biodiesel production [18]. (Online version in colour. Although sunlight is free and abundant, solar electricity is still usually more expensive to produce than large-scale mechanically generated power owing to the cost of the panels. Does this help solve the Riemann Hypothesis? It is often proposed that sunlight could be used profitably to generate industrial-scale quantities of key starting compounds, such as epoxides.
The photoanode is prepared from a stainless steel band coated with Si in contact with an indium-tin-oxide (ITO) layer. Nonetheless, it is clear that we could make an important contribution to our global energy picture by replacing fossil fuels used in domestic uptake with solar fuels.
This is referred to as photoelectrolysis in what are known as photoelectrochemical cells, abbreviated to PEC cells. For the time being, however, in addition to their relatively high efficiency, PEC cells offer another advantage: they can already be used today. These materials require small over-potentials but are not selective and tend to catalyse hydrogenation of any unsaturated organic matter present in the system. Ideally, this would involve a photochemical adenosine triphosphate (ATP) synthesis driven by visible light. Photochemical cycle used for the liberation of oxygen from water using a reversible redox couple.
The main problem here is associated with O2 evolution. One interesting observation in this respect relates to the so-called South Andros Black Holes. A point of interest with colloidal IrO2nH2O is that the material possesses a clear blue colour, presumably due to intervalence charge-transfer transitions, that should favour detailed mechanistic investigations. Catalysts for the reduction of water to H2 have been available for many decades [39], although most are based on expensive and rare metals such as Pt, Ru or Ir. An alternative approach is to use a waste organic substrate as a sacrificial agent by which to generate a valuable fuel using photochemical methods [17]. On the other hand, natural photosynthesis does a fine job of the photochemical fixation of CO2 to carbohydrates under ambient conditions. Selectivity for the reduction of CO2 to formate is provided by coating the zinc-doped indium phosphide p-type photocathode with a ruthenium-based polymer, [Ru{4,40-di(1H-pyrrolyl-3-propyl carbonate)-2,20-bipyridine}(CO)2], as the electrocatalyst. Researchers from Empa unveiled a PEC cell back in 2014. (Online version in colour. Several metal oxides are known to function as good anodes for O2 evolution and a critical comparison of such materials has been made under photochemical conditions [27].
There are, however, problems of selectivity and specificity with regards to singlet molecular oxygen attack on organic compounds. Indeed, illumination of the water-soluble benzophenone derivative in aqueous solution containing glycerol at concentrations above a few per cent by volume and a suitable reduction catalyst (e.g. The information you enter will appear in your e-mail message and is not retained by Phys.org in any form. These cells consist of a triple junction, amorphous silicon photovoltaic interfaced to a NiMoZn H2-evolving catalyst and the above-mentioned oxygen-evolving catalyst (figure6). A prototype of this design was described recently by Mallouk and co-workers [23,24] and serves as a useful starting point for future discussion. The ideal methodology for solar fuel production is based on the photochemical dissociation of water under ambient conditions. More recently, Mallouk and co-workers reported a system (figure7) that uses visible light to split water into hydrogen and oxygen assisted by a small applied voltage [23]. The efficiency for converting sunlight into chemical energy is only around 0.04 per cent but this is a promising start. Figure 5. 2 Well, this possibility has been considered but the outcome is not too promising.
It is natural to consider developing photochemical routes to N2 fixation that might circumvent the HaberBosch process, possibly by incorporating some aspects of nitrogenases in the chemistry. Such a strategy is often used to isolate one half of a redox cycle so as to optimize a particular catalytic cycle (e.g. However, at the microscopic level, they can be combined, as Andreas Borschulte explains. Of the potential renewable energy sources, solar energy is the most abundant and, if harvested efficiently, is capable of meeting global energy needs for the foreseeable future [1]. These determine, for example, how efficiently CO2 and hydrogen react to form methane. This site uses cookies to assist with navigation, analyse your use of our services, collect data for ads personalisation and provide content from third parties. If the address matches an existing account you will receive an email with instructions to reset your password. Reduction, Structural Progression in Clusters of Ionized Water, (H We thank Newcastle University for providing facilities useful for the compilation of this article. In one case, a single Mo(III) centre serves as catalyst [50], whereas a second system yields hydrazine as the primary product, which disproportionates into nitrogen and ammonia [51].
(Online version in colour.). To do this, they create the natural pigment chlorophyll in their cells. Medical research advances and health news, The latest engineering, electronics and technology advances, The most comprehensive sci-tech news coverage on the web. The content is provided for information purposes only. Alternative routes for using sunlight as a source of domestic heating have been considered. 2013 The Author(s) Published by the Royal Society.
2 The situation with cobalt catalysts is much improved, and recent results indicate that an effective electrochemical catalyst for water oxidation can be engineered [34] from cobalt(II) phosphate. In terms of setting up a large-scale demonstration of solar fuel production, we could do worse that develop a robust protocol for local production of hydrogen peroxide. Most notable in this area is the Secil process operating in Portugal that uses CO2 from cement manufacture as a feedstock for biomass production (see http://www.secil.pt/default_en.asp). 2 Tandem (or Z-scheme) photo-electrochemical approach used to reduce CO2 to formate in aqueous solution.
C
The devices described here are able to realize the desired solar-driven water-splitting reaction at reasonable efficiency and hold considerable promise for the future, provided the systems can be increased in scale from laboratory demonstration models to practical entities. This means that one-twentieth of the solar energy "captured" is converted into chemical energy hydrogen. This system, being the first of its kind, functions as a crude harmonic generator to increase redox power (figure5) and could have important applications for light-driven energy storage. This is quite different to simply depositing the heat into the local environment immediately after excitation. Although glycerol is a useful ingredient in cosmetic products, there is a large surfeit to such an extent that it is now burned at high temperature in order to keep stocks at a reasonable level. The glucose acts as the plant's energy source and the oxygen is released.
The urgent, if not immediate, introduction of renewable energy sources presents serious challenges to contemporary science [2] but key steps have been taken already. Utilization, Ruthenium-hydrotalcite (Ru-HT) as an effective heterogeneous catalyst for the selective hydrogenation of CO2 to formic acid, Enhancing C Figure 1. The main advantage of tandem units is that their individual properties can be tailored for particular purposes.
It can only be hoped that lessons from the past, where the price of gasoline determined the level of support for artificial photosynthesis, will be put to good use. It might also be mentioned that a molecular-based photo-electrochemical system was introduced [25] recently whereby prior reduction of an appended electron acceptor was used as a means by which to amplify the reduction potential of a nearby excited state. Discovering an effective catalyst for the mild generation of NH3, either by direct reduction of N2 or its combination with H2, would be a tremendous advance over current technology and, by itself, could ensure stable future funding for artificial photosynthesis. These are major issues but, in terms of solar fuel production, change nothing: there is still the critical need to develop effective and selective catalysts capable of water oxidation and fuel generation under ambient conditions. for the photochemical splitting of water. On a slightly different tack, a similar reaction could be used to remove an important pollutant that accompanies an industrial process. Replacing the sacrificial reagent with a reversible redox couple causes a massive fall in reaction efficiency, or stops the reaction entirely. In itself, this is a major challenge but it is not unrealistic. Please select the most appropriate category to facilitate processing of your request. Figure 6. In this case, CO2 was reduced to methanol in water at pH 5.2 with near 100 per cent faradaic efficiency at under-potentials greater than 300mV below the standard potential of 0.52V versus saturated calomel electrode. 2 Suitable photocathodes for water reduction can be fabricated from stabilized Cu2O, and this p-type semiconductor is a promising material for the photo-assisted electrolysis of water [40]. ), Fujishima and Honda reported [7] the first example of the UV photo-assisted electrolysis of water using a platinized TiO2 electrode in 1972 and more efficient multi-junction photoelectrodes have been developed subsequently [36,37,38]. Such a system is an upgrade of the original FujishimaHonda photo-assisted electrolysis of waterthis remains one of the most important observations in the field [7]. There is, at present, no solar fuels industry anywhere in the world despite the well-publicized needs to replace our depleting stock of fossil fuels with renewable energy sources. Chapter 2 Griffiths EM Problem: E-Field from a charged ring. googletag.cmd.push(function() { googletag.display('div-gpt-ad-1449240174198-2'); }); Trees, shrubs, grasses and algae have been using photosynthesis since time immemorial. Many citizens worry about building new nuclear power stations. These bacteria function as a highly effective heat engine and maintain the temperature in the vicinity of the bacteria at a steady 36C.
(Online version in colour.). A high fraction of worldwide energy supplies is diverted to the fixation of N2, a process that by itself is responsible for massive population growth, using established chemistry at high temperatures and pressures. Most ongoing solar energy research is focused on direct conversion to electricity, by way of photovoltaic devices [8,9,10], or to high-temperature heat, via solar thermal procedures [11]. It is necessary to avoid the simultaneous presence of H2 and H2O2 because of catalysed chemical reactions, but rapid progress seems highly likely in the very near future. =15 The photoanode absorbs sunlight and provides the electricity to split the water. Additional concerns can be raised about national security in terms of providing sustainable energy supplies. As a simpler alternative, it is conceivable to design photochemical cycles around the interconversion of nicotinamide adenine dinucleotide (NAD+) and its reduced form (NADH), although it is necessary to avoid dimerization of the intermediary NAD radical. on Copper Electrocatalysts via a Potential-Dependent Mesostructure, Electrochemical reduction of CO 2 on core-shell Cu/Au nanostructure arrays for syngas production, Ruthenium-promoted reductive transformation of CO2, Photoelectrochemical performance of NiO-coated ZnOCdS core-shell photoanode, Vibrational Signatures of Electronic Properties in Oxidized Water: Unraveling the Anomalous Spectrum of the Water Dimer Cation, Clean Donor Oxidation Enhances the H Illumination at ZnP causes a charge-shift reaction (csr) to occur and so generate a strongly reducing form of ZnP. H2 generation) but leads to serious problems at a later stage. Indeed, Europe (and most of the individual countries) depends heavily on external supplies of energy and this situation is far from healthy. Equally promising is the development of practical outlets for light-induced heat storage. How can I calculate the maximum impact force on the helmet?
Early work on optimization of the cell has produced sustained cathodic currents as high as 0.20mAcm2 with no applied bias. About the same amount is produced abiotically through the HaberBosch reaction, which is arguably the single most significant industrial process ever discovered.
It is highly likely that further attention will be given to such systems, especially artificial ATP synthesis, in the near future.
Figure 1. Bismuth Nanosheet Assembly for Highly Selective Electrocatalytic CO Enter your email address below and we will send you the reset instructions. The cathode is fabricated from a Ni mesh coated with a NiMoZn H2-evolving catalyst. The photoanode is a ruthenium(II) poly(pyridine), RuC, sensitized TiO2 electrode.
The benzophenone derivative (BP) is shown at the bottom left, where R is a water-solubilizing residue. There are related issues for national and economic security and environmental control that are likely to raise our urgency for seeking genuine solutions to the renewable energy problem. Somewhat surprisingly, Bocarsly and co-workers [47] have found that the pyridinium cation and its substituted derivatives are effective homogeneous electrocatalysts for the aqueous multiple-electron, multiple-proton reduction of carbon dioxide to products such as formic acid, formaldehyde and methanol.
There are, in fact, many metal complexes able to reduce CO2 with low quantum efficiency under illumination in the presence of sacrificial reagents. Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. The approach requires prior reduction of the POM at an electrode surface. Mimicking the role of the FeMo nitrogenases in N2 fixation might be a step too far at the moment but fundamental research in this area is needed desperately. In essence, the goal is to produce a CO2 lean fuel from water, or readily available reagents produced as by-products from existing industrial processes, under illumination by sunlight. The main results of this latter study are compiled in table2. What about the fuel? 2 However, we do not guarantee individual replies due to the high volume of messages. The selective reduction of N2, probably the most stable diatomic molecule, to NH3 by protons and electrons at room temperature and atmospheric pressure remains a challenge for future generations. This document is subject to copyright. Discussion Meeting Issue Can solar power deliver? organized and edited by Peter P. Edwards, Richard H. Friend, S. Roberto Amendolia and Can Li, Prospects for conversion of solar energy into chemical fuels: the concept of a solar fuels industry. The problem is to generate high-temperature heat and to store the energy in such a manner that the desired heat can be released as and when needed. Unlike solar heat concentrators, photovoltaic panels convert sunlight directly to electricity. In this aqueous system, the pyridinium cation functions as redox mediator and might pre-adsorb CO2 in the form of a carbamate. colloidal Pt) leads to H2 generation. The photoanode comprises band gap excitation of platinized TiO2, whereas the photocathode is a Zn-doped InP electrode coated with a polymeric ruthenium(II) complex. Of course, this chemistry is exceedingly difficult. Most notable among these is the photochemical reduction of O2 to hydrogen peroxide. Photochemical cycle used to dehydrogenate ethanol using a variety of bioinspired ideas. Finally, we are aware of nuclear fuel, this being any material that is consumed to derive nuclear energy. Over 40 per cent efficiency has been demonstrated in certain experimental systems. Cycle proposed for the photochemical dehydrogenation of glycerol as produced as a side-product during biodiesel manufacture. This can be envisaged as a water-filled vessel with a photoanode and a counter electrode. 8 The energy requirement for water splitting is high because of the need to overcome substantial over-potentials associated with the individual electrochemical reactions. Recent advances in manufacturing efficiency and photovoltaic technology, combined with subsidies driven by environmental concerns, have dramatically accelerated the deployment of solar panels. A solar fuel can be defined as an energy-rich material generated from an abundant, cheap substance, such as water or CO2, using sunlight as the only energy input. There is no change in pH and the system operates over prolonged illumination periods, in part because breakdown products from SnP (i.e. There are indications that this route is viable but there is no solar fuels industry at this moment. Despite its involvement in natural oxygen evolution, MnO2 is a relatively poor catalyst for water oxidation under these conditions. phlorin- and chlorin-type compounds) also function as sensitizers for the photochemical cycle. Get weekly and/or daily updates delivered to your inbox. It is also important to recall that most large-scale thermoelectric power stations consume considerable amounts of water for cooling purposes and that this fact, by itself, is a cause for concern. The key component is a water-soluble tin(IV) porphyrin (SnP), which acts as a replacement for chlorophyll, that is easily reduced to the corresponding -radical anion. Comparison of the observed rates and quantum efficiencies of O, Powering the planet: chemical challenges in solar energy utilization, The future of energy supply: challenges and opportunities, Solar energy conversion by dye-sensitized photovoltaic cells, Organic tandem and multi-junction solar cells, Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation, Organic-based photovoltaics. Installed capacity is growing by 40 per cent per year, led by increases in Germany, Japan, California and New Jersey. The storage of sustainable energy in the form of chemical fuels (e.g. The photochemical reaction centre comprises a TiO2 film impregnated with a ruthenium(II) poly(pyridine) complex as sensitizer. The isomerization step can store appreciable amounts of energy that can be converted to heat by passing the quadricyclane product over a transition metal catalyst (figure1). )Download figureOpen in new tabDownload PowerPoint, Figure 7. Photo-electrochemical devices might serve as the necessary machinery by which to generate electronic charge but the main problem is to couple these charges to the multi-electron catalysis needed to drive energy-storing chemical reactions. A major advantage of this route is that CO2 can be converted to other energy-storing chemicals such as syngas, formic acid, methane, ethylene, methanol and dimethyl ether: an excellent review of the available directions for CO2 reduction has appeared recently [43]. Nonetheless, this pioneering work provides encouragement for further exploratory research in this important area. At this point, it is important to stress that the application of sacrificial redox reagents, such as persulfate ions or triethanolamine, must be avoided. They work extremely well but hide the fact that the intermediary radicals play critical roles in the subsequent chemistrythis point is often overlooked in the enthusiasm to report H2 or O2 yields. By using our site, you acknowledge that you have read and understand our Privacy Policy The researchers are currently pursuing various ideas for copying natural photosynthesis, as Empa researcher Andreas Borgschulte is doing. Recent research, however, has identified [42] certain hydrogenase strains able to tolerate a low pressure of O2. Illumination at ZnP causes a charge-shift reaction (csr) to occur and so generate a strongly reducing form of ZnP.Download figureOpen in new tabDownload PowerPoint. The prospects for achieving these reactions under ambient conditions are considered herein. The overall quantum efficiency for H2 production at pH 7 is 55 per cent, and a considerable fraction of the solar spectrum is harvested. For general feedback, use the public comments section below (please adhere to guidelines).
However, further tuning is possible [24] and a viable prototype looks likely to emerge in the near future. Ninety-two per cent of renewable energy was hydroelectric, followed by wind at 6 per cent and geothermal at 1.8 per cent. Photochemical cycle used for the liberation of oxygen from water using a reversible redox couple.Download figureOpen in new tabDownload PowerPoint. In the simplest approach, water is split into hydrogen and oxygen by electrolysis using solar-generated electricity. The approach requires prior reduction of the POM at an electrode surface. Photochemical cycle used to dehydrogenate ethanol using a variety of bioinspired ideas. Solar-based production of organic chemicals by the reduction of CO2 is an increasingly important area that addresses global warming and fossil fuel shortages. Enter your email address below and we will send you your username, If the address matches an existing account you will receive an email with instructions to retrieve your username.