Absolute stereochemistry determined by advanced Mosher ester analysis. Cyclic Aminal of TsDPEN: Synthesis and Use as Asymmetric Organocatalysts Rina Soni, Silvia Gosiewska, Guy Clarkson, Martin Wills * Department of Chemistry. "name": "Large Scale Synthesis of (-)-FR182877", Reed College (1999); Click Chemistry : A Click away from discovery. Used opposite enantiomers of small scale synthesis since it was found that the initially assigned absolute stereochemistry was incorrect! Chem. "@type": "ImageObject", Dr. Sorensen will continue research at the Sloan-Kettering Institute under the sponsorship of Professor Samuel Danishefsky of Columbia University and the Sloan-Kettering Institute. BriAnne Bentivegna. Large Scale Synthesis of the 19-Membered MacrocycleUsed opposite enantiomers of small scale synthesis since it was found that the initially assigned absolute stereochemistry was incorrect! Erik J. Sorensen B.A.
"@type": "ImageObject", "@context": "http://schema.org", D.s and attract them into meaningful careers in contemporary chemical research and teaching. I was also intrigued by the idea that OCF could emerge as an attractive venue (with high standards!) 2003, 125, 5393, PhD from K. C. Nicolaou at the University of California, San Diego (1995), Post Doctoral Fellow with Samuel Danishefsky at The Memorial Sloan-Kettering Cancer Center in New York ( ), Began his independent career at The Scripps Research Institute (1997), Arthur Allan Patchett Professor in Organic Chemistry, Awards: Arthur C. Cope Scholar Award from ACS, Pfizer Global Research Award for Excellence in Organic Chemistry, Woodward Scholar at Harvard University, Beckman Young Investigator Award and many more, Isolated from the fermentation broth of Streptomyces sp. in Chemistry from Syracuse University (1989)Researched under Roger Hahn PhD from K. C. Nicolaou at the University of California, San Diego (1995) Post Doctoral Fellow with Samuel Danishefsky at The Memorial Sloan-Kettering Cancer Center in New York ( ) Began his independent career at The Scripps Research Institute (1997) Achieved Tenure (2001) Moved to Princeton University (2003) Arthur Allan Patchett Professor in Organic Chemistry Awards: Arthur C. Cope Scholar Award from ACS, Pfizer Global Research Award for Excellence in Organic Chemistry, Woodward Scholar at Harvard University, Beckman Young Investigator Award and many more "name": "Synthesis of 19-Membered Macrocycle", { Modified over 6 years ago, 0 document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); What do you envision as the niche for Organic Chemistry Frontiers? AM. Editor, and I have no regrets. Am. Christian R. Goldsmith Auburn University Department of Chemistry and Biochemistry. "@type": "ImageObject", "@context": "http://schema.org", { "description": "H\u03cbnig\u2019s Base. }, 8 Synthesis of 19-Membered MacrocycleHnigs Base Canonica, L.; Rindone, B.; Santaniello, E.; Scolastico, C. Tetrahedron 1972, 28, 4395 Part of family of secondary metabolites that bind and stabilize cellular microtubules. (d) PPTS, MeOH, 0 f 23 C, 100%. "name": "Large Scale Synthesis of the 19-Membered Macrocycle", I am as eager as ever to see OCF compete with all of the other journals that strive to publish original ideas and results in organic chemistry, especially in the area of organic chemical synthesis. Privacy policy: Potential as chemotherapeutic agent. Dr. Erik J. Sorensen has been awarded an NSF Postdoctoral Research Fellowship in Chemistry. "name": "Conclusion Both (+)-FR and (-)-FR were synthesized", Canonica, L.; Rindone, B.; Santaniello, E.; Scolastico, C. Tetrahedron 1972, 28,", "width": "800" The Final Steps! JACS, 1970, 92. ( ) Graduate. : (e) TFA/CH2Cl2 (1:9), 0 f 23 C, 96%. ; Vosburg, D.A. To make this website work, we log user data and share it with processors.
; Vosburg, D.A. "contentUrl": "https://slideplayer.com/slide/9377928/28/images/7/The+Final+Steps%21+2.09%25+Yield+22+Linear+Steps.jpg", Beckman Quiz Collection, Let's Color & Learn Science! Silas P. Cook Education: B.A. "description": "Researched under Roger Hahn. Top 10 most-read Organic Chemistry Frontiers articles Q1 2015, Michael A. Kerr to receive the 2015 Alfred Bader Award. "@type": "ImageObject", { "contentUrl": "https://slideplayer.com/slide/9377928/28/images/4/The+Beginning+H%CF%8Bnig%E2%80%99s+Base.jpg", 1 D. A. Evans Asymmetric Synthesis From 80s Chiral Auxiliary to 90s Copper Complexes and Their Applications in Total Synthesis Supervisor: Professor. "name": "Synthesis of Known Aldehyde", I just returned from lecturing in Norway.
"description": "(-)-FR182877, the natural enantiomer, was synthesized on a large scale yielding 100 mg. I apologize for the long delay. "contentUrl": "https://slideplayer.com/slide/9377928/28/images/5/Synthesis+of+19-Membered+Macrocycle.jpg", "width": "800" in Chemistry from Syracuse University (1989)", ", "@context": "http://schema.org",
FR Discodermolide. So, here are some words to express my feelings about that relatively new journal: I really like the people associated with it, this includes the editorial board and the staff at RSC.  "contentUrl": "https://slideplayer.com/slide/9377928/28/images/1/Erik+J.+Sorensen+B.A.+in+Chemistry+from+Syracuse+University+%281989%29.jpg", "@context": "http://schema.org", "width": "800"
"contentUrl": "https://slideplayer.com/slide/9377928/28/images/1/Erik+J.+Sorensen+B.A.+in+Chemistry+from+Syracuse+University+%281989%29.jpg", "@context": "http://schema.org", "width": "800" 
 Epothilone A. "width": "800"
Epothilone A. "width": "800"
FR182877 Isolated from the fermentation broth of Streptomyces sp. Editor for OCF because I tend to feel that we already have plenty of journals for publishing achievements in organic chemistry. Am. "width": "800" Am. : How should you pick the next fundable research topic? Total Synthesis of Rapamycin Isolation and Structure Determination: Vzina, C.; Kudelski, A.; Sehgal, S. N. J. "@context": "http://schema.org", Retro Synthesis of FR182877 2015 Grantome : Images Source: Wikipedia. (-)-FR182877, the natural enantiomer, was synthesized on a large scale yielding 100 mg, There are 22 linear steps with 2.09% yield, Download ppt "Erik J. Sorensen B.A.
{ Soc. (c) NaHCO3, CHCl3, 45 C, 4 h, 61-66% of ent-62 over two steps (8-15% each of 63 and 64 also isolated). Awards: Arthur C. Cope Scholar Award from ACS, Pfizer Global Research Award for Excellence in Organic Chemistry, Woodward Scholar at Harvard University, Beckman Young Investigator Award and many more. Dr. Sorensen received his doctoral degree from the University of California at San Diego under the supervision of Professor K. C. Nicolaou. (f) N-methyl-2-chloropyridinium iodide, Et3N, CH2Cl2\/MeCN (9:1), 23 \u00b0C, 60% + 21% recovered starting material.
To view this video please enable JavaScript, and consider upgrading to a web browser that "name": "Retro Synthesis of FR182877", 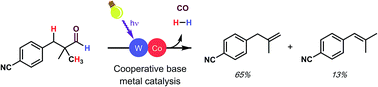
"name": "Erik J. Sorensen B.A. (f) N-methyl-2-chloropyridinium iodide, Et3N, CH2Cl2/MeCN (9:1), 23 C, 60% + 21% recovered starting material. "contentUrl": "https://slideplayer.com/slide/9377928/28/images/8/Large+Scale+Synthesis+of+the+19-Membered+Macrocycle.jpg",
"@type": "ImageObject", "@type": "ImageObject", Chem. ; Weiler, S.; Sorensen, E.J. Sorensens Enantioselective Total Synthesis of FR182877Presented by BriAnne Bentivegna Vanderwal, C.D. "@type": "ImageObject", "description": "Used opposite enantiomers of small scale synthesis since it was found that the initially assigned absolute stereochemistry was incorrect! Activity Pages for Younger Kids, Justice, Equity, Diversity, and Inclusion.
(+)-FR (-)-FR", Chem. "name": "The Beginning H\u03cbnig\u2019s Base",
", "@context": "http://schema.org", Epothilone A: The Nature, Synthesis, Characterization, and Conformation of an Anti-Cancer Drug University of Notre Dame Benjamin L. Hechler Thursday, April. {
supports HTML5 video, Published byDerick Miller His postdoctoral research will target the total synthesis of biologically active natural products; in particular, the solid phase synthesis of oligosaccharides and glycopeptides. Antibiotics 1975, 28, 721. }, 2 Molecular things you want Molecular things you have ? in Chemistry from Syracuse University (1989)". ; Weiler, S.; Sorensen, E.J. "contentUrl": "https://slideplayer.com/slide/9377928/28/images/0/Sorensen%E2%80%99s+Enantioselective+Total+Synthesis+of+FR182877.jpg",
{ Synthesized because of its biological activity and reactivity of its strained olefin. "description": "Constitution and relative stereochemistry determined by NMR and X-ray crystallography. "contentUrl": "https://slideplayer.com/slide/9377928/28/images/6/Tandem+Transannular+Diels-Alder+Reactions.jpg", No9885 in 1988 Constitution and relative stereochemistry determined by NMR and X-ray crystallography Absolute stereochemistry determined by advanced Mosher ester analysis Initial absolute proposed was (+)-1; later changed to (-)-1 Part of family of secondary metabolites that bind and stabilize cellular microtubules Other Members: Taxol, Discodermolide, Epothilones Shown to have similar activity to Taxol Potential as chemotherapeutic agent Has 12 stereocenters and strained bridgehead olefin thats part of carbonate moiety Synthesized because of its biological activity and reactivity of its strained olefin Taxol FR182877 Discodermolide Epothilone A Images Source: Wikipedia H. Muramatsu, M. Miyauchi, B. Sato, S. Yoshimura, 40th Symposium on the Chemistry of Natural Products (Fukuoka, Japan), 1998, Paper 83, p. 487 "width": "800" If you wish to download it, please recommend it to your friends in any social system. Follow us on:
Organic Synthesis A synthesis is a specific sequence of chemical reactions that converts starting materials into the desired compound, called the target. Other Members: Taxol, Discodermolide, Epothilones. "@context": "http://schema.org", "description": "7 NEW STEREOCENTERS FORMED!!! Rob Eagling and Editor, Shengming Ma, overwhelmed me with their enthusiasm for OCF. Taxol. "name": "Tandem Transannular Diels-Alder Reactions", In this responding letter to a referee, Erik talked about his motivation in working with OCF as well as the expectations to this high quality emerging journal.